Flavin protein reduced by succinate – Flavin proteins, reduced by succinate, are an essential component of the electron transport chain, playing a crucial role in cellular respiration. Their intricate structure and mechanism, as well as their involvement in various biological processes, make them a fascinating subject of study.
This article delves into the structure, mechanism, and clinical significance of flavin proteins reduced by succinate, exploring their diverse functions and potential therapeutic applications.
Flavoprotein Structure and Mechanism
Flavoproteins are proteins that contain a flavin cofactor, which is a derivative of riboflavin (vitamin B2). The flavin cofactor is involved in a variety of redox reactions, including the transfer of electrons from succinate to oxygen.The structure of flavin proteins varies depending on the specific protein, but all flavin proteins contain a flavin cofactor that is bound to the protein by non-covalent interactions.
The flavin cofactor is typically located in a hydrophobic pocket within the protein, and it is surrounded by amino acid residues that help to stabilize the cofactor and to orient it for catalysis.The mechanism of flavin reduction by succinate is a two-step process.
In the first step, succinate is oxidized to fumarate, and the electrons from succinate are transferred to the flavin cofactor. In the second step, the reduced flavin cofactor is oxidized by oxygen, and the electrons from the flavin cofactor are transferred to oxygen.The
overall reaction for the reduction of flavin by succinate is as follows:“`Succinate + FAD → FADH2 + Fumarate“`The reduction of flavin by succinate is an important step in the citric acid cycle, which is a central metabolic pathway in all aerobic organisms.
The citric acid cycle is responsible for the generation of ATP, which is the cell’s main energy currency.
Succinate Dehydrogenase Complex
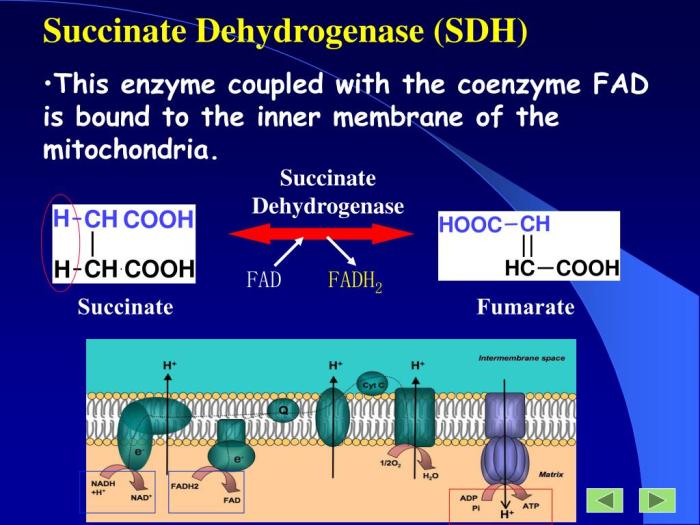
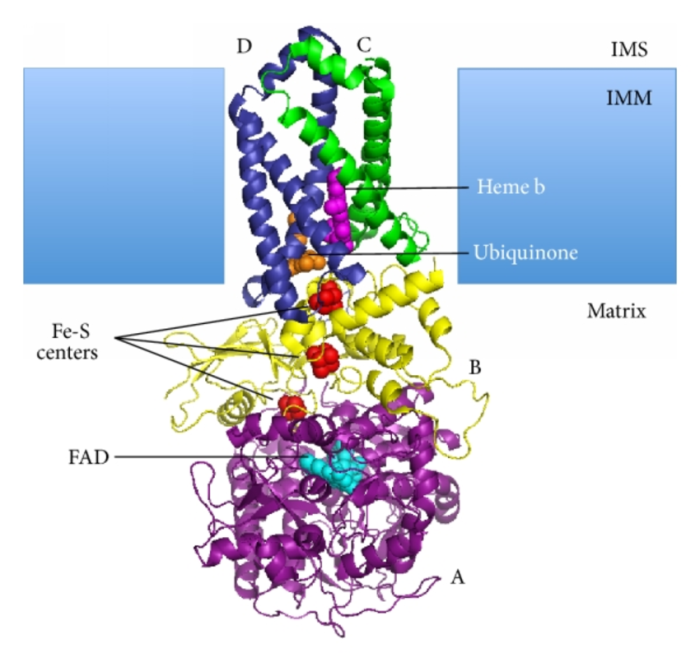
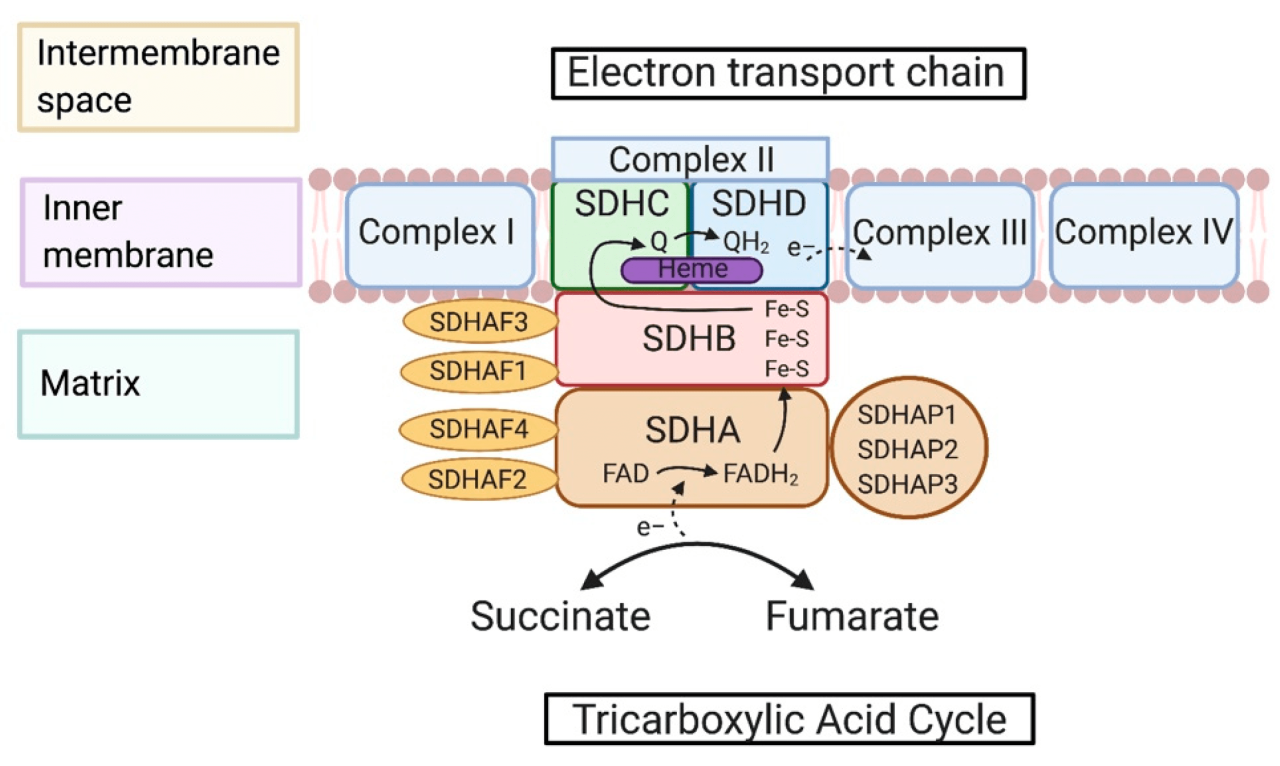
The succinate dehydrogenase complex (SDH) is a membrane-bound multi-enzyme complex that plays a crucial role in the citric acid cycle, also known as the Krebs cycle. It is located in the inner mitochondrial membrane and catalyzes the oxidation of succinate to fumarate, a key step in energy production.The
SDH complex is composed of four subunits:
- Flavoprotein (Fp): Contains a flavin adenine dinucleotide (FAD) cofactor that accepts electrons from succinate.
- Iron-sulfur protein (ISP): Contains three iron-sulfur clusters that transfer electrons from Fp to ubiquinone.
- Cytochrome b560: A membrane-bound cytochrome that transfers electrons from ISP to ubiquinone.
- Hydrophobic protein: Anchors the complex to the mitochondrial membrane.
Role of Flavin Protein in the Complex
The flavin protein (Fp) is a critical component of the SDH complex. It contains a FAD cofactor that undergoes a two-electron reduction during the oxidation of succinate. The reduced FAD then transfers the electrons to the iron-sulfur protein (ISP), initiating the electron transfer chain within the complex.The
Fp also plays a role in the assembly of the SDH complex. It interacts with the other subunits, ensuring their proper orientation and stability. Mutations in the Fp gene can lead to defects in SDH complex assembly and function, resulting in various disorders, including Leigh syndrome and mitochondrial encephalopathy.
Flavin-Dependent Enzymes
Flavin-dependent enzymes are a group of enzymes that utilize flavin cofactors to catalyze various biochemical reactions. These enzymes play crucial roles in cellular metabolism, energy production, and redox reactions.Flavin cofactors are derivatives of riboflavin (vitamin B2) and exist in two main forms: flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD).
Flavin cofactors undergo reversible redox reactions, allowing them to participate in electron transfer processes.
Examples of Flavin-Dependent Enzymes
Numerous flavin-dependent enzymes are involved in a wide range of biological processes. Some notable examples include:
- Succinate dehydrogenase:A key enzyme in the citric acid cycle, catalyzing the oxidation of succinate to fumarate.
- NADH-cytochrome c reductase:A component of the mitochondrial electron transport chain, transferring electrons from NADH to cytochrome c.
- Monoamine oxidase:Involved in the metabolism of neurotransmitters, catalyzing the oxidative deamination of monoamines.
- Glutathione reductase:Protects cells from oxidative stress by reducing oxidized glutathione (GSSG) to its reduced form (GSH).
- Cytochrome P450 enzymes:A family of enzymes involved in drug metabolism and detoxification.
Functions and Biological Significance
Flavin-dependent enzymes play essential roles in cellular metabolism and energy production. They participate in electron transfer reactions, generating reducing equivalents (NADH and FADH2) that drive ATP synthesis in the respiratory chain. Additionally, these enzymes are involved in various metabolic pathways, including the citric acid cycle, amino acid metabolism, and detoxification processes.Disruptions
in the activity or regulation of flavin-dependent enzymes can lead to various diseases and disorders. For example, defects in succinate dehydrogenase have been associated with mitochondrial encephalopathies, while mutations in monoamine oxidase genes have been implicated in neuropsychiatric disorders.
Clinical Significance: Flavin Protein Reduced By Succinate
Flavin proteins play crucial roles in various metabolic pathways and cellular processes, and their dysfunction can lead to several diseases.Flavin proteins are involved in the electron transport chain, which generates energy for cells. Defects in flavin proteins can impair cellular respiration, leading to conditions such as mitochondrial disorders and neurodegenerative diseases.
For example, mutations in the flavin-containing succinate dehydrogenase complex can cause Leigh syndrome, a severe neurodegenerative disorder.
Diagnostics
Flavin proteins can be used as biomarkers for certain diseases. For instance, measuring the activity of flavin-containing enzymes, such as lactate dehydrogenase, can aid in the diagnosis of liver and heart diseases.
Therapeutics
Flavin proteins are being explored as potential therapeutic targets for various diseases. For example, inhibitors of flavin-dependent enzymes are being investigated as potential treatments for cancer and parasitic infections.
Experimental Techniques
Investigating flavin proteins requires a diverse range of experimental techniques. These methods encompass both spectroscopic and biochemical approaches, each offering unique insights into the structure, function, and dynamics of these proteins.
Spectroscopic Methods
Spectroscopic techniques exploit the unique optical properties of flavin cofactors to probe their electronic and conformational states. Key methods include:
- UV-Visible Spectroscopy:Measures the absorption and emission of light by flavin cofactors, providing information about their redox state and electronic transitions.
- Fluorescence Spectroscopy:Detects the emission of light by excited flavin cofactors, offering insights into their conformational changes and protein-flavin interactions.
- Electron Paramagnetic Resonance (EPR) Spectroscopy:Probes the unpaired electrons in flavin semiquinone radicals, revealing their spin states and molecular dynamics.
Biochemical Methods, Flavin protein reduced by succinate
Biochemical techniques complement spectroscopic methods by assessing the enzymatic activity, protein-protein interactions, and molecular dynamics of flavin proteins. Prominent methods include:
- Enzyme Assays:Measure the catalytic activity of flavin proteins, providing insights into their substrate specificity, kinetic parameters, and reaction mechanisms.
- Protein-Protein Interaction Studies:Identify and characterize the interactions between flavin proteins and other proteins, shedding light on their assembly and function.
- Molecular Dynamics Simulations:Utilize computational methods to model the dynamic behavior of flavin proteins, providing atomic-level insights into their conformational changes and functional mechanisms.
By combining these experimental techniques, researchers can comprehensively characterize flavin proteins, unraveling their intricate roles in biological processes.
Commonly Asked Questions
What is the role of flavin proteins in the electron transport chain?
Flavin proteins act as electron carriers, transferring electrons from succinate to coenzyme Q in the electron transport chain.
How are flavin proteins reduced by succinate?
Flavin proteins are reduced by succinate through a specific enzymatic reaction catalyzed by succinate dehydrogenase.
What are the clinical implications of flavin protein deficiencies?
Flavin protein deficiencies can lead to various clinical conditions, including neurological disorders and metabolic abnormalities.