Delving into the intricacies of acid-base chemistry, this conjugate acid base pair worksheet embarks on a journey to unravel the fundamental concepts and applications of conjugate acid-base pairs. By delving into their properties, reactions, and significance, we gain a deeper understanding of the chemical world around us.
Conjugate acid-base pairs, intertwined in a delicate dance, play a pivotal role in numerous chemical processes. This worksheet unravels the intricacies of their relationship, shedding light on their behavior and impact on various systems.
Conjugate Acid-Base Pair Definition
In chemistry, a conjugate acid-base pair refers to two species that can interconvert through the transfer of a proton (H+ ion). When an acid donates a proton, it transforms into its conjugate base, and when a base accepts a proton, it transforms into its conjugate acid.
For example, in the reaction between hydrochloric acid (HCl) and water (H2O), HCl donates a proton to H2O, forming the conjugate base chloride ion (Cl-) and the conjugate acid hydronium ion (H3O+):
- HCl (acid) + H2O (base) → Cl- (conjugate base) + H3O+ (conjugate acid)
Acid and Base Properties
An acid is a substance that donates protons (H+ ions), while a base is a substance that accepts protons. The strength of an acid is determined by its tendency to donate protons, and the strength of a base is determined by its tendency to accept protons.
The stronger the acid, the weaker its conjugate base, and vice versa. This is because the more readily an acid donates a proton, the less readily its conjugate base can accept a proton.
Conjugate Acid-Base Reactions
Conjugate acid-base reactions are reactions that involve the transfer of a proton between a conjugate acid and a conjugate base. These reactions are typically reversible, meaning that they can proceed in both directions.
The position of equilibrium in a conjugate acid-base reaction is determined by the relative strengths of the acid and base involved. The stronger the acid, the more the reaction will proceed towards the formation of its conjugate base, and vice versa.
Acid-Base Equilibria
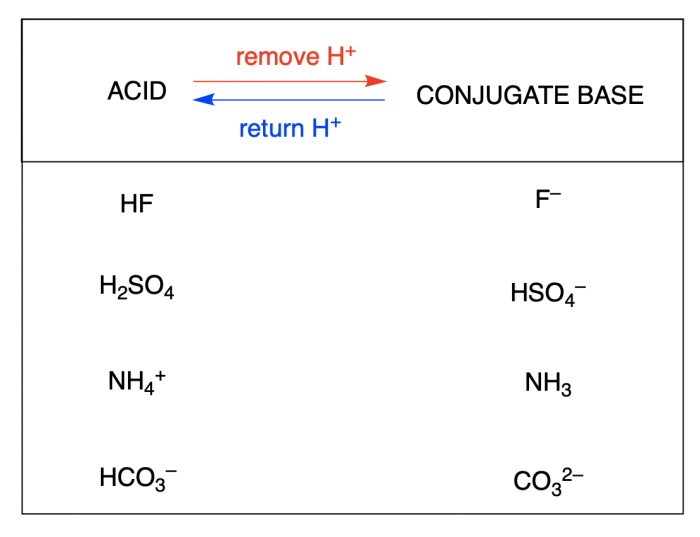
Acid-base equilibria are systems in which the concentrations of acids and bases remain constant over time. This occurs when the forward and reverse reactions in a conjugate acid-base reaction are occurring at the same rate.
The position of acid-base equilibria is affected by several factors, including the temperature, the concentration of the acid and base, and the presence of other ions in the solution.
pH and Conjugate Acid-Base Pairs: Conjugate Acid Base Pair Worksheet

The pH of a solution is a measure of its acidity or alkalinity. It is determined by the concentration of hydrogen ions (H+) in the solution.
Conjugate acid-base pairs can be used to buffer solutions, which means that they can help to maintain a constant pH. This is because the conjugate acid and base can react with each other to neutralize any changes in the pH.
Applications of Conjugate Acid-Base Pairs
Conjugate acid-base pairs have a wide range of applications in everyday life and in biological systems.
- In everyday life, conjugate acid-base pairs are used in batteries, fertilizers, and food preservatives.
- In biological systems, conjugate acid-base pairs are involved in many important processes, such as the regulation of pH, the transport of molecules across cell membranes, and the catalysis of enzymatic reactions.
Q&A
What is a conjugate acid-base pair?
A conjugate acid-base pair consists of two species that differ by the transfer of a single proton (H+ ion). When an acid donates a proton, its conjugate base is formed, and when a base accepts a proton, its conjugate acid is formed.
How does pH affect conjugate acid-base pairs?
pH is a measure of the acidity or basicity of a solution. A low pH indicates a high concentration of H+ ions (acidic), while a high pH indicates a low concentration of H+ ions (basic). The pH of a solution can influence the equilibrium position of a conjugate acid-base pair, favoring the formation of the species that is more stable under the given pH conditions.